Which of the Following Elements Has the Smallest Ionic Radius
Helium has the smallest atomic radius. More shielding of the electrons by the highest occupied energy level.
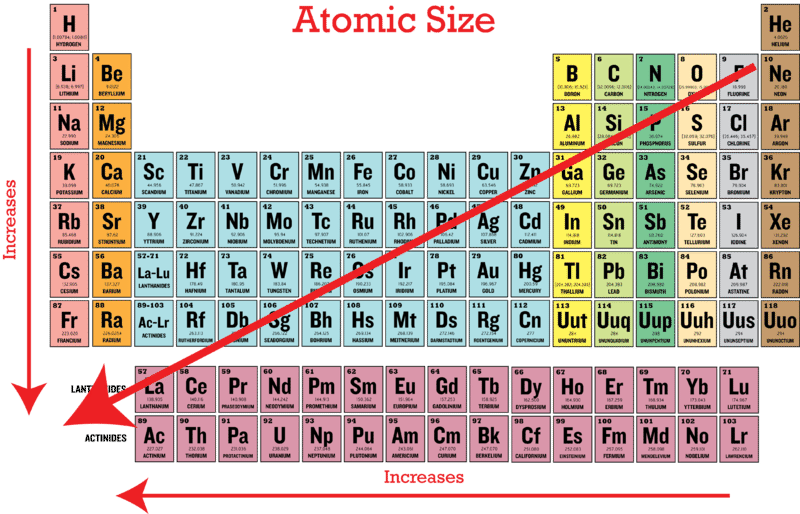
Periodic Trends In Atomic Size Ck 12 Foundation
B 3.

. Solve any question of The d and f Block Elements with-Patterns of problems Was this answer helpful. Upon ionization it forms the ion Li. Pm 3 Easy.
Correct option is C As we move from left to right in a raw radii decreases. But we came up with a problem. Solve any question of The d and f Block Elements with-Patterns of problems Was this answer helpful.
Which of the following elements has the smallest ionic radius Date22 February 2017 Author. Pm 3 Easy. The distance from the center of the nucleus to the most probable location of.
Admin It is quite important to understand the line following problem first. Which of the following factors contributes to the increase in atomic size within a group in the periodic table as the atomic number increases. Calcium has a larger atomic radius than Magnesium.
Which Element Has The Largest Radius Na Or Cs. Lu 3 D. Therefore the increasing order of radii.
Dy 3 C. Which Element Has The Largest Radius Na Or Cs. This is due to trends in the periodic table and the effective nuclear charge that holds the valence electrons close to the nucleus.
Helium has the smallest atomic radius. What element in the second period has the largest atomic radius. 13 Which of the following species has the smallest ionic radius.
Answerthanks for the free points dear Which of the following elements has the smallest ionic radius. A argon B neon C helium D krypton 6 Using shorthand nation the ground sae election conualin to 7. This is due to trends in the periodic table and the effective nuclear charge that holds the valence electrons close to the nucleus.
Nd 3 B. Prečo sa atómový polomer v priebehu periódy zmenšuje. This means the only electron it has is in.
Of the following which element has the highest first ionization energy. Which of the following has the smallest ionic radii. Atomic radius decreases as you move across a period from left to right and decreases as you move up a group from bottom to top.
Correct option is C As we move from left to right in a raw radii decreases. Prečo sa atómový polomer zmenšuje zľava doprava. Which has larger radius mg or CA.
What is the charge. Which of the following elements has smallest radius. Prečo je atómový polomer neónu väčší ako fluór.
Which of the following elements has the smallest atomic radius. This means it has lost the electron in the valence shell probably through an ionic transfer. A Li B Be 2 C B 3 D Li 2 Medium Solution Verified by Toppr Correct option is C Li Be 2andB 3 are isoelectronic species.
Ako vypočítate atómový polomer. As can be seen in the figures below the atomic radius increases from top to bottom in a group and decreases from left to right across a period. Thus helium is the smallest element and francium is the largest.
Which of the following elements has the smallest. Ktorý má väčší atómový polomer Na alebo MG. Simple CleverBut if you dont just do like me.
4 Which ion has the smallest ionic radius. Which of the following has smallest ionic radius. Which of the following elements has the smallest ionic radius.
Lu 3 D. By Get Answers The Boss 181k points181k points 32 233 823 asked in Other Jan 11 5. A Li B Na C K D Rb 5.
Lithium has 3 electrons 2 belonging to the k shell with the valence L having the other electron. So radii of L u 3 is smallest among all. Dy 3 C.
A Al3 B Na C Mg2 D S2-E Cl-. So if your white is and your black is. Hence the form univalent positive ions after ionization.
Aký je rozdiel medzi atómovým polomerom a atómovou veľkosťou. The ion that has 28 protons and 26 electrons is 8The number of electrons in the ion Zn is. The group one elements ionize by losing one electron.
So radii of L u 3 is smallest among all. Nd 3 B. Which of the following has smallest ionic radius.
Which element has the smallest atomic radius. In such a series size decreases as the effective nuclear charge atomic number of the ion increases.
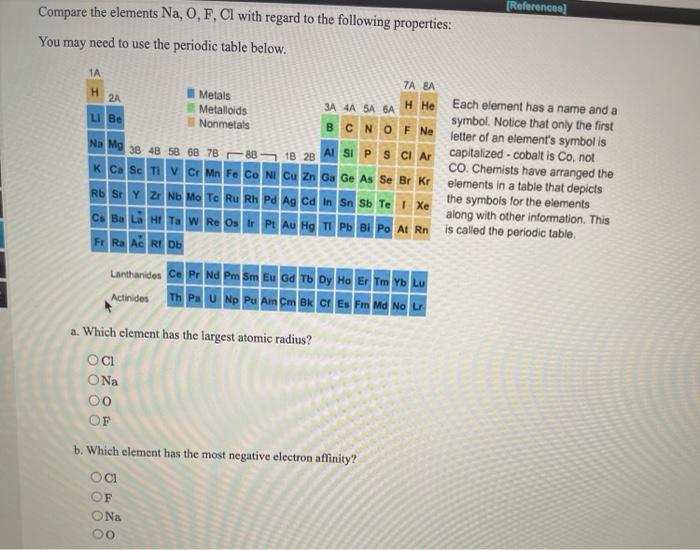
Solved A In The Following Set Which Atom Has The Smallest Chegg Com


No comments for "Which of the Following Elements Has the Smallest Ionic Radius"
Post a Comment